Chapter 9: Safety
Cryogenic liquids present significant but familiar and manageable hazards because of their intense cold and substantial gas production when warmed. There are three principal areas of hazard: physiological, physical and chemical. Physiological hazards include frostbite, respiratory ailments and the specific effects of certain materials such as oxidisers on body tissues. Physical hazards include the effects of over-pressure, low temperatures, oxygen enrichment and cryogenic embrittlement. Chemical hazards include the ignition of flammable mixtures, the propagation of flame and explosions.
However, the risks of handling cryogenic liquids can be minimised using standard safety equipment and well understood procedures. The operational experience of Highview Power Storage further suggests that even the most significant hazard of liquid air – oxygen enrichment – can be managed effectively and safely. The safety record of the industrial gases industry more generally has improved dramatically over the past 40 years and is now comparable to that of many world class companies.
1. Overview of hazards related to liquid air
Since liquid air is not yet produced in commercial volumes, less health and safety literature has been published than for its separated constituents: liquid oxygen and liquid nitrogen. However, three big players in the cryogenic distillation field - Air Liquide, Air Products and Praxair - have produced Material Safety Data Sheets (MSDS) for this product (see Appendix 3). These describe liquid air as a refrigerated, non-toxic, non-flammable gas that presents cold burns/frost bite, oxidation and strong support of combustion as the principle hazards.
The general rules for distribution and transportation of liquid air are similar to other cryogenic fluids, and purpose designed cryogenic equipment is required. Transport of large quantities of liquid air may be by road tanker, ship or pipeline. Smaller quantities are conveniently handled in portable Dewars, cylinders and other insulated vessels. In all cases distribution must be accomplished in complete safety and with minimum loss of liquid air. There are many established documents from leading cryogenic companies, such as those mentioned earlier, about large scale cryogen transport by road tanker and pipeline that could be applied to liquid air.
The hazards associated with liquid air, liquid oxygen and liquid nitrogen are compared in Table 9.1. The relative severity of the hazard is represented by the number of Xs. The potential hazards must all be considered individually and collectively in the design and operation of any cryogenic system.

Table 9.1: Relative severity of cryogenic fluid hazards
In most areas of potential risk, the hazards associated with liquid air are more severe than those of liquid nitrogen but less than those of liquid oxygen, which is highly reactive. Comparing liquid air with liquid nitrogen, many hazards, such as cold effects, and over-pressure effects are the same, but in one respect liquid air is safer than liquid nitrogen.
When liquid cryogens are expelled into the atmosphere at room temperature, they will evaporate and expand ~700-800 times their liquid volume very rapidly. In the case of liquid nitrogen, even small amounts of liquid can displace large amounts of air and decrease the oxygen content of the atmosphere below a safe level, raising the possibility of asphyxiation. In the case of liquid air, since it is made up of oxygen and nitrogen in the same proportion as the atmosphere, its evaporation will produce oxygen gas as well as nitrogen. Provided there is adequate ventilation, this will not cause the same level of oxygen deficiency as the evaporation of liquid nitrogen.
For liquid air, additional care must be taken against the possibility of oxygen enrichment and associated chemical hazards. Oxygen enrichment occurs because nitrogen, oxygen and argon have different partial vapour pressures at the same temperature. As vapour pressure is the driving force for both condensation and evaporation of a mixture of gases, a selective phase change process can take place over time and oxygen enrichment of liquid air is to be expected. Therefore we suggest handling of liquid air should follow many of the procedures for handling liquid oxygen, which are well understood and have been in operation in the industrial gases industry for decades.
2. Specific hazards and mitigation
Most of the health and safety issues of liquid air can be referred to established protocols relating to liquid nitrogen and liquid oxygen. The most likely issues relating to the use of liquid air or liquid nitrogen in the power and energy sectors are:
- Cold burn or frostbite (both).
- Pressure build-up (both).
- BLEVE (Boiling Liquid Expanding Vapour Explosion) (both).
- Materials structure and integrity (both).
- Oxygen deficiency (mainly liquid nitrogen).
- Oxygen enrichment (mainly liquid air).
Cold burn or frostbite
The extremely low temperatures of cryogenic liquids mean that liquid, cold vapour or gas, can produce serious health problems. In general, frostbite occurs only after prolonged exposure of tissue to temperatures below 0C. Because blood delivers heat to the affected part, the amount of heat actually removed from the tissues and the rate at which it is removed determine the extent of frostbite when it occurs. Other cold hazards include:
- Contact of the skin with cryogenic liquids (or even cold gas) can cause severe cryogenic burns.
- Contact with non-insulated and even insulated parts of equipment or vessels containing cryogenic liquids can produce similar damage - eg cold pipework and other surfaces.
- Inhalation of cold vapour can reduce the blood temperature, cause damage to the lungs and trigger asthma attacks.
- Hypothermia is a risk, depending on the length of exposure, the atmospheric temperature and the individual. The internal organs are cooled by the blood from the outer parts of the body. If the heart and brain are cooled to any great extent it can be fatal.
Though potentially serious, these hazards can be mitigated by proper insulation, protective clothing and suitable ventilation for those working in close proximity/contact with cryogenic fluids, pipework, containers and gases. Protective measures should include:
- Protective clothing for handling low temperature liquefied gases serves mainly to protect against cold burns.
- Non-absorbent gloves (leather or PVC) should always be worn when handling anything that is in contact with liquid air or its cold vapour. Gloves should be a loose fit so that they can be easily removed should liquid splash on or into them.
- If there is a risk of severe spraying or splashing, eyes should be protected with a face shield or goggles.
- Trousers should be worn outside boots and have no pockets.
A number of health and safety documents have been produced and procedures are well established for handling different cryogenic fluids.
Pressure build-up
As cryogens are usually stored at or near their boiling point, there is always some gas present in the container, so the high pressure gas hazard is always present. Upon phase change, cryogenic liquids vaporise with a volume expansion to ~700-800 times. The rate of evaporation will vary, depending on the characteristics of the fluid, container design, insulating materials, and environmental conditions. Without adequate venting or pressure-relief devices on containers, large pressures can build up on cryogen evaporation. In extreme cases this can lead to Boiling Liquid Expanding Vapour Explosion (BLEVE).
Due to the large temperature difference between cryogen and ambient temperature, heat flux into the cryogen is unavoidable regardless of the quality of the insulation. Cryogens boil as they sit in their storage vessels or transfer pipes by absorbing heat from the surroundings. Since cryogenic fluids have small latent heats and large expansion ratios, even a small heat input can create large pressure increases, especially in a confined space. Cryogenic Dewars lose roughly ~1% of their contents to evaporation per day.
A number of situations can cause high pressure to build-up. Measures including good ventilation, pressure relief valves, venting lid and proper operations are needed to prevent an explosion. Pressurisation can occur because:
- Ice forms on the venting tube, plugging it and preventing gas release.
- Equipment damage results in cryogenic fluids leaking into small areas, where the cryogenic liquid vaporises and causes pressure build up.
- A cryostat or Dewar loses vacuum.
- Direct contact of the cryogenic liquid with water or some other ambient liquid in a tube may result in rapid vaporisation of the cryogenic liquid and can cause the tube to explode.
A number of common measures are used to reduce these risks, which include:
- Pressure relief devices must be provided on each and every part of a cryogenic system. Satisfactory operation of these devices must be checked periodically and may not be defeated or modified at any time.
- Vents must be protected against icing and plugging. Vents must be kept open at all times.
These are well established measures that are currently incorporated into cryogenic container and system design.
Boiling Liquid Expanding Vapour Explosion
Boiling Liquid Expanding Vapour Explosion (BLEVE) is an explosion caused by the rupture of a vessel containing any pressurised liquid above its boiling point, and typically occurs when the tank is exposed to fire. As pressure increases a point is reached when the walls of the container can no longer withstand the pressure and the vessel bursts. This produces instantaneous depressurisation, meaning the temperature of the liquid will be higher than that which would correspond to it according to the saturation curve on a Pressure-Temperature diagram, and the liquid will be superheated. As a result, homogeneous nucleation takes place in the liquid, which will rapidly and continuously generate vapour that accelerates the explosion process.
BLEVE can happen with any liquid, whether flammable or not, but the greatest hazard is with flammable liquids such as liquefied natural gas (LNG), where a number of accidents during transport have been reported.1 When BLEVE occurs in non-flammable cryogenic gases such as liquid air or nitrogen the hazard is less severe since it produces no combustion.
Measures to mitigate the risk of BLEVE include:
- Install pressure relief valves so that if tanks are subjected to external heat they will vent cryogen harmlessly to the atmosphere before the pressure rises to dangerous levels.
- Use tanks with good fire resistance, drawing on experience from the LNG industry.
- Keep any combustible material away from tanks.
Low temperature effect on materials structure and integrity
As the properties of most materials are altered with decreases in temperature, the design of cryogenic systems and prevention of hazards requires knowledge of the strength, thermal expansion, thermal conductivity and heat capacity of the construction materials over the operational temperature range. Materials which are normally ductile at atmospheric temperatures may become extremely brittle when subjected to temperatures in the cryogenic range, while other materials may improve their ductility.
Materials must be carefully selected for cryogenic service because of the drastic changes in the properties of materials when they are exposed to extremely low temperatures. Cryogenic liquids can cause many common materials such as carbon steel, some types of plastics and rubber to become brittle, or even fracture under stress. Some metals which are suitable for cryogenic temperatures include stainless steel (300 series and other austenitic series), copper, brass, bronze, monel, and aluminum. Non-metal materials which perform satisfactorily in low temperature service are Dacron, Teflon, Kel-F and asbestos impregnated with Teflon, Mylar and nylon.2
Oxygen deficiency (mainly for liquid nitrogen)
Oxygen deficiency is defined as the condition of the partial pressure of atmospheric oxygen being less that 135 mmHg.3 At higher altitudes the same effects generally occur at greater volume concentrations since the partial pressure of oxygen is less. If exposure to reduced oxygen is terminated early enough, effects are generally reversible. If not, permanent central nervous system damage or death can result.
Respiratory ailment effects are a risk in enclosed areas. However, risks can be mitigated through use of oxygen concentration meters and proper ventilation. Health and Safety procedures for the amount of ventilation required for an environment with cryogenic inert gasses are well established. In general, there are well established measures and precautions to deal with oxygen deficiency hazard (ODH).4
Oxygen enrichment (mainly for liquid air)
Air is composed primarily of nitrogen, oxygen and argon, which have different partial vapour pressures at the same temperature. As it is a mixture, the gases can be separated by contact with cold surfaces. The equilibrium composition diagram for nitrogen and oxygen is shown in Figure 9.1. As the temperature of mixture A (air, 21% oxygen and 79% nitrogen) is lowered, a condensate first appears at the dew point, approximately 82K (-191C). The composition of this condensate is approximately 50% oxygen and 50% nitrogen. These equilibrium values are approached by the condensate that forms on uninsulated lines and other exposed solid and liquid surfaces at temperature below 82K. When liquid B boils at 1 atm, the vapor contains approximately 6% oxygen and 94% nitrogen so the liquid phase residues slowly becomes enriched with oxygen. The enrichment rate decreases as the liquid oxygen concentration increases. The last part of the liquid to be evaporated contains approximately 50% oxygen. Thus with air, oxygen enrichment can be expected in the liquid phase as a result of both the condensation and vaporisation processes.
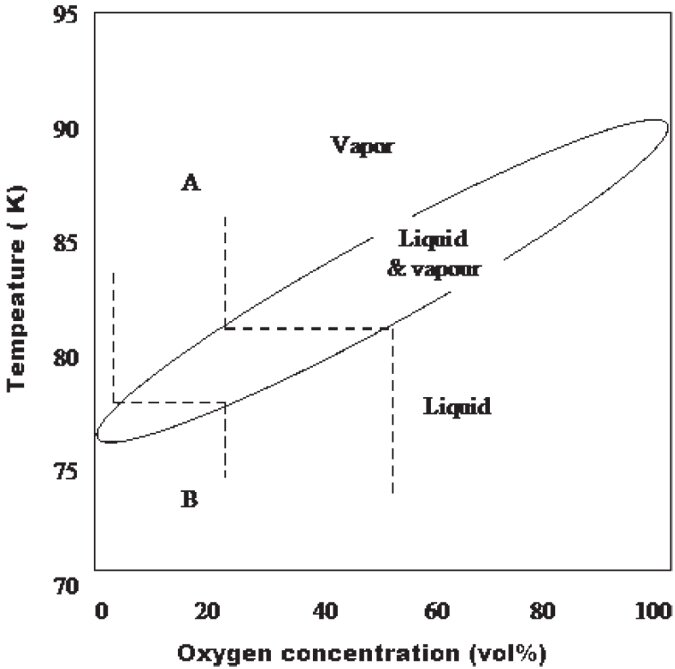
Figure 9.1: Oxygen and nitrogen phase diagram
As evaporation makes liquid air progressively richer in oxygen, potentially as high as 50%, we suggest for safety reasons that in circumstances where liquid air may be stored for long periods it should be handled according to liquid oxygen handling procedures. The MSDS documents for handling pure liquid oxygen from Air Liquide and BOC appear in Appendix 3.
The causes of oxygen enrichment include:
- Condensation by cooler cryogens: When transferring liquid nitrogen through uninsulated metal pipes, the air surrounding a cryogen containment system can condense. This can cause oxygen enrichment on the surface or entrapment in unsuspected areas.
- Condensation on cold metal surface: Extremely cold metal surfaces are also capable of condensing oxygen from the atmosphere.
- Evaporation of liquid air: As explained above, liquefied inert gases such as liquid nitrogen which have a lower boiling point than oxygen, will evaporate first, and this could lead to oxygen enrichment up to 50%.
Measures to minimise the risk of oxygen enrichment include:
- Use properly insulated systems.
- Monitor the oxygen content of liquid air.
- Adopt active measures such as systems proposed by Stirling Cryogenics and Refrigeration5 and BOC.6
- Keeping organic materials such as oil, grease, kerosene, cloth, wood, paint, tar and dirt away from the oxygen source as they may combust in an oxygen rich environment.
- In the event that air is condensed and some repair work is needed, special care must be taken especially where the use of open flames or other potential sources of ignition is intended.
- Installing equipment that reduces oxygen enrichment, such as the systems developed by Stirling Cryogenics and Refrigeration and BOC.7
3. Managing oxygen enrichment – Highview Power Storage
The potential of oxygen enrichment within the storage tank of a liquid air energy system such as the Highview pilot plant in Slough presents a real but controllable risk, particularly if the system is operated for reserve and peaking services where the storage period is greater than several weeks.
Significant oxygen enrichment within the bulk storage vessel of a cryogenic energy storage device would present increased operational hazards in both the storage and power recovery phases. This would limit the suitability of the technology and significantly increase its installation and operating costs.
Operational experience gained from Highview’s pilot plant suggests that localised enrichment in stagnant pipes can occur after five to seven days, but concentrations were shown to return to normal levels once lines were purged from the tank, indicating no significant enrichment within the bulk liquid. No further indications of oxygen enrichment were observed within the bulk liquid.
The potential of oxygen enrichment within the cryogenic storage vessel is likely to be lower in larger tanks – where the higher ratio of volume to surface area favours the retention of cold - and those where the storage period is measured in hours or days.
Previous solutions
Previous solutions to the problem of oxygen enrichment (e.g. patents US5,571,231 and US3,260,060) broadly use cold from low pressure streams, such as the venting stream, to condense the gaseous vapour and stabilise the concentration of the liquid. However, not all cryogenic energy storage vessels are equipped with such a gaseous venting stream.
Operational experience at Highview Power Storage
The pilot plant in Slough uses an industry standard, vacuum insulated steel cryogenic storage tank of about 60 tonnes capacity. Apart from standard equipment supplied by the manufacturer to monitor fluid level and pressure in the tank, instrumentation was limited to monitoring the composition of the fill and discharge streams. The tank and cryogenic pumps were cleaned to oxygen handling standards and the process stream on the suction side of the pumps was closely monitored to ensure oxygen concentrations did not exceed 23% while the power recovery turbine was in operation. During the performance testing programme, marginally elevated oxygen levels were noted at sample points in the discharge line after extended down periods (>five to seven days, depending on atmospheric conditions), and this was most noticeable while the tank was less than half full. However, by simply extending the pre-run pump cooling cycle times by 50%, oxygen concentration was observed to return to normal (21%). This suggests the enrichment had occurred in the stagnant lines, where thermal losses are higher, resulting in locally elevated liquid temperature and not in the bulk storage tank.
Implications for commercial-scale plants
An energy storage device can provide a wide variety services to the network operator, generators and consumers of power, ranging from daily balancing to rarely-called back-up capacity. The nature of each service will influence the size and duration of the storage required, and this in turn has implications for the management of the oxygen enrichment hazard.
There are two types of liquid air plant: the Cryo Energy System (CES), where liquid air is produced, stored and used to generate power on a single site; and the Cryogenset, which is a generation-only device that runs on cryogens delivered from a remote or centralised production site.
The Cryogenset would typically be used to provide emergency back-up for companies and/or peaking plant for the grid, both of which would probably involve the storage of relatively small amounts of cryogen for weeks and possibly months depending on market and infrastructure conditions. As a result the risk of oxygen enrichment would be higher, and it would make sense to eliminate the risk entirely by running the plant on liquid nitrogen rather than liquid air. This might make the ‘fuel’ more expensive, but since the plant would run so infrequently – typically less than 100 hours per year – it should not critically undermine the economics. As discussed in chapter 6, there is estimated to be an 8,500tpd daily surplus of nitrogen gas available for liquefaction.
A full-scale Cryogenic Energy System, on the other hand, could perform a variety of roles with different implications for oxygen enrichment. A CES plant used for shaping inflexible nuclear power or firming intermittent renewable generation is likely to be cycling at least once a day, meaning the storage period would be far too short for any significant oxygen enrichment to occur – a point supported by operational experience of theSlough plant. A commercial-scale CES would also typically have larger storage tanks with proportionally lower thermal losses, further reducing the risks.
A CES plant running on liquid air would be most at risk of oxygen enrichment if providing a reserve or peaking service. However, the risk is thought to be low because of the operating schedule, which would involve long periods of charging followed by relatively short periods of discharge. This is because the liquefaction plant - if correctly sized for the application - should take almost the entire period between discharges to fully charge the store. Assuming the plant is running at a constant rate, this in turn means the effective storage period of the total stored capacity is only half the total storage period. It also means the vessel is no longer full of stagnant cryogenic fluids for a long period, but continually being mixed with sub-cooled liquid air, which would help to reduce boil off and any oxygen enrichment.
4. Hazards and mitigation in transport
The hazards associated with liquid air or liquid nitrogen are fundamentally identical in static and transport applications. However, since these cryogens are not currently widely used as transport fuel, some further discussion is warranted. The hazards and potential mitigation measures relating to some key transport-specific situations are summarised in Table 9.2.
In transport, the risk of cold burn would be greatest during refuelling, either from spillage or contact with cold surfaces. However, this risk can be eliminated by design, using adequate insulation and locking fuel hoses, as it has been at LNG refuelling stations for heavy trucks.8 The industrial gas company Messer has developed a refuelling system for cryogenic nitrogen called Ecolin, for which the data sheet appears in Appendix 4.
The risk of pressure build-up and explosion would be highest if the vacuum insulation of a vehicle fuel tank were penetrated during a collision, but this could be mitigated with appropriate pressure-relief valves and burst disks.
The risk of asphyxiation would be highest if a vehicle fuelled on liquid nitrogen were left idle in an enclosed space such as a garage for an extended period. However, this hazard could be mitigated by legislating appropriate passive ventilation standards, oxygen monitoring equipment or both.
The risk of oxygen enrichment would be higher in the fuel tanks of vehicles left standing for an extended period than for large static tanks (see section 3), since the lower volume to surface area ratio means small tanks do not retain their cold so well. This hazard could be eliminated using technical solutions such as the AirLock System proposed by Stirling Cryogenics9 or the system for storing a multi-component cryogenic fluid proposed by BOC.10

Table 9.2: Hazards and mitigation in transport applications
More generally, there is good reason to believe the hazards associated with the use of liquid air and liquid nitrogen as transport fuel can be managed to acceptable levels, because:
- The industrial gas industry transports thousands of tonnes of cryogenic liquids by road tanker daily (see section 5, and chapters 6 and 7).
- LNG and LPG is increasingly used as lorry fuel, and the hazards of transporting liquid air are expected to be much lower than for either LNG/LPG or for liquid oxygen, which is also commonly transported by road.
- Early applications of liquid air in transport are likely to involve commercial vehicles with fully trained operators.
- Hazardous fuels such as petrol and diesel are routinely used by the public and the hazards have been managed to acceptable levels.
5. The safety record of the industrial gas producers
The safety record of industrial gas producers in Europe has improved dramatically over the past forty years. Data supplied by the European Industrial Gas Association (EIGA) shows the Lost Time Injury Rate (LTIR) has fallen from around 30 days lost per million hours worked in 1978 to just 1.7 in 2011 (Figure 9.2). That compares well to the performance of the wider chemicals industry of which the industrial gases business is a sector. The last published figures showed an LTIR of 6.6 for the European chemicals industry in 2008 and 4.57 for the global chemicals industry (Figure 9.3).

Figure 9.2: EIGA LTIR 1977-2012. Source: EIGA12

Figure 9.3: LTIR comparison between EIGA and related industries and companies. Source: EIGA13
EIGA data also shows that injuries in the industrial gases industry were largely related to routine workplace hazards – over 40% were due to trips and falls, falling from height, and over exertion – while far fewer were caused by cryogenic hazards. 3.5% of injuries were due to exposure to heat or cold, while 5.5% were caused by fire, energy release or flying particles (Figure 9.4).

Figure 9.4: EIGA LTIR accidents by cause. Source: EIGA14
EIGA’s LTIR performance is worse than that of the international oil and gas industry, which stood at 0.43 in 2011, but its Fatal Accident Rate (FAR) is much lower. The oil industry’s 10 year average FAR is 3.6 per 100 million man hours worked per year, against 1.7 for EIGA.15
6. Conclusions
This survey of safety and liquid air suggests the following conclusions:
- Any use of liquid air or liquid nitrogen as an energy vector presents serious but familiar and manageable hazards of cold, oxygen enrichment and asphyxiation.
- These hazards are well understood and subject to standard industry safety procedures, regulations and equipment.
- The experience of Highview Power Storage in managing oxygen enrichment is encouraging, as is the safety record of the industrial gases industry.
- Safety considerations may suggest liquid air is preferable to liquid nitrogen in some circumstances and vice versa.
- Any use of liquid air or liquid nitrogen by members of the public would require it to be as safe or safer than using petrol or diesel, and all relevant technologies would need to be designed and engineered to ensure this.
- There is no insuperable safety reason why liquid air and/or liquid nitrogen should not be widely deployed as an energy vector in both grid and transport applications.
Endnotes
1
Potential for BLEVE associated with marine LNG vessel fires, Pitblado, Journal of Hazardous Materials, 140, 2006, pp527-5346.
2
Boiler and Pressure Vessel Code, Section VIII Unfired Pressure Vessels, American Society of Mechanical Engineers, 2010, http://files.asme.org/Catalog/Codes/PrintBook/32477.pdf
3
Cryogenic Safety Manual, Argonne National Laboratory, http://www.phy.anl.gov/division/esh/Cryogenic/Appendix%203/Appendix%203.htm
4
See for example Oxygen Hazard Safety booklet from Jefferson Lab, http://www.jlab.org/accel/safetylb/ODH-book.pdf
5
The AirLock System, preventing O2 enrichment in liquid air vessels, Stirling Cryogenics and Refrigeration,
6
Apparatus for storing a multi-component cryogenic liquid, US Patent 5571231A, BOC,
7
Ibid.
9
The AirLock System, preventing O2 enrichment in liquid air vessels, Stirling Cryogenics and Refrigeration,
1 0
Apparatus for storing a multi-component cryogenic liquid, US Patent 5571231A, BOC,
12
http://www.eiga.eu/index.php?id=158
13
Ibid.
14
Ibid.
15
Safety performance indicators - 2011 data, International Organization of Oil & Gas Producers (OGP), http://www.ogp.org.uk/pubs/2011s.pdf; and EIGA personal communication, February 2013.
